Your Describe rutherfords gold foil experiment images are available in this site. Describe rutherfords gold foil experiment are a topic that is being searched for and liked by netizens now. You can Get the Describe rutherfords gold foil experiment files here. Download all royalty-free vectors.
If you’re looking for describe rutherfords gold foil experiment images information related to the describe rutherfords gold foil experiment keyword, you have pay a visit to the right blog. Our site always gives you suggestions for viewing the highest quality video and picture content, please kindly surf and find more informative video articles and images that fit your interests.
Describe Rutherfords Gold Foil Experiment. This chemistry video tutorial provides a basic introduction into Rutherfords Gold Foil Experiment. The electron was discovered by JJ. His experiment caused him to reject Thomsons atomic model who was. Rutherford in his experiment directed high energy streams of α-particles from a radioactive source at a thin sheet 100 nm thickness of gold.

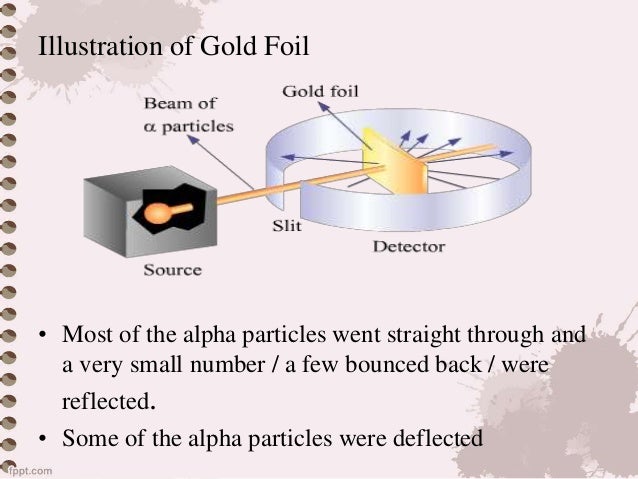
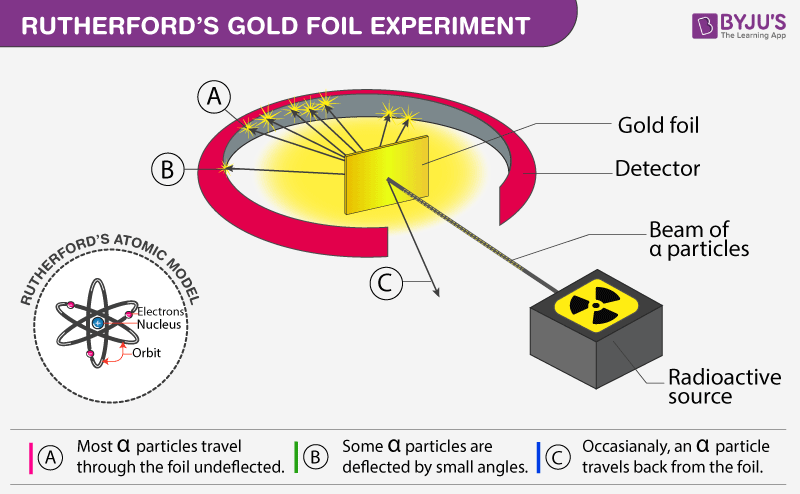
Alpha particles are fired at thin gold foil. Most of the beams went through the foil but a few were deflected. Describe Rutherfords gold foil experiment. Photographic film is placed around the foil. The alpha particles that were fired at the gold foil were positively charged. By bombarding a very thin gold foil with alpha particles Hans Geiger and Ernest Marsden both students of Rutherford observed that a small fraction 1 in 8000 of these particles were deflected at large angle as if it bounced off a heavy obstacle.
If Thomson was correct the beam would go straight through the gold foil.
They took very thin sheets of gold foil only 4 105 cm thick and bombarded it with a stream of alpha a particles. Two of his students Hans Geiger and Ernest Marsden an undergraduate set out to measure the number of alpha particles. Alpha particles are fired at thin gold foil. Here are the steps that were completed. Rutherfords gold foil experiment Rutherfords alpha particle scattering experiment refers. Rutherford overturned Thomsons model in 1911 with his famous gold-foil experiment in which he demonstrated that the atom has a tiny massive nucleus.
 Source: study.com
Source: study.com
Other areas were scattered around the film and even back by the alpha particle source. The Rutherford Experiment. The Rutherford Scattering Experiment. Rutherford overturned Thomsons model in 1911 with his famous gold-foil experiment in which he demonstrated that the atom has a tiny massive nucleus. See full answer below.
 Source: slideshare.net
Source: slideshare.net
When Rutherford shot α particles through gold foil he found that most of the particles went through. The Rutherford Experiment. The alpha particles that were fired at the gold foil were positively charged. The existence of protons was also known as was the fact that atoms were neutral in charge. Rutherford and his co-workers made a fundamental contribution in understanding the structure of the atom and establishing the presence of a small nucleus in the atom.
 Source: sciencefacts.net
Source: sciencefacts.net
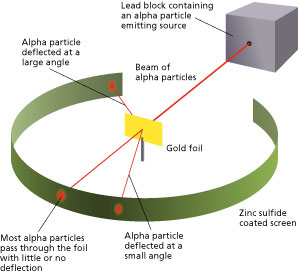
They took very thin sheets of gold foil only 4 105 cm thick and bombarded it with a stream of alpha a particles. Tony Tyson April 22 2013. Since the intact atom had no net charge and the electron and proton had opposite charges the next step after the discovery of subatomic particles. Principle of Rutherfords experiment. Ernest Rutherford performed the gold foil experiment to propose the atomic model.
 Source: large.stanford.edu
Source: large.stanford.edu
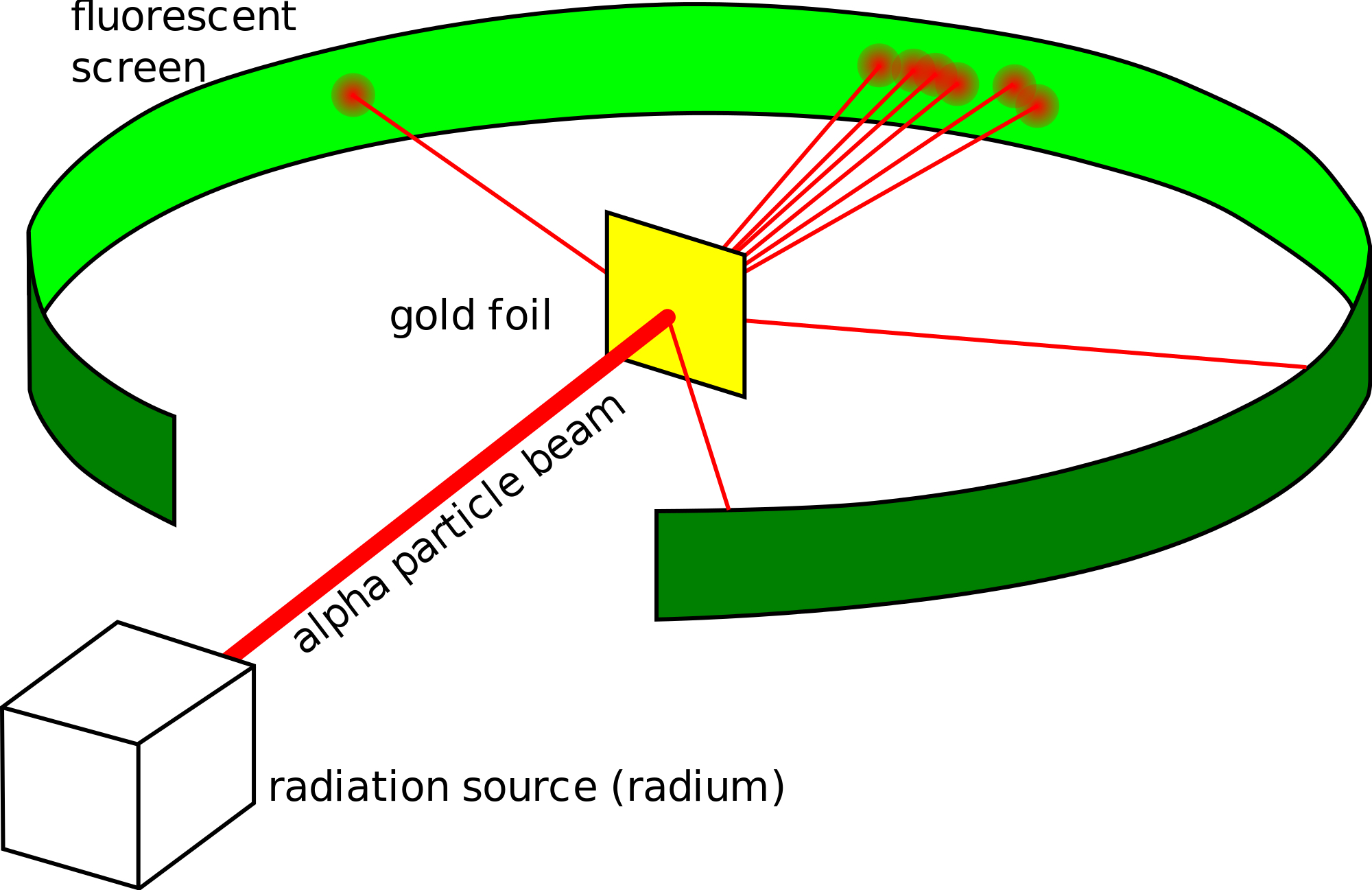
Rutherfords conducted an experiment by bombarding a thin sheet of gold with α-particles and then studied the trajectory of these particles after their interaction with the gold foil. Rutherford designed an experiment to use the alpha particles emitted by a radioactive element as probes to the unseen world of atomic structure. He beamed a ray of alpha particles onto a gold foil and. Use the microscope to observe different parts of the screen and look for. Describe Rutherfords gold foil experiment.
 Source: expii.com
Source: expii.com
Aug 8 2014. The electron was discovered by JJ. See full answer below. Some scattered in various directions and a few were even deflected back towards the source. Rutherford in his experiment directed high energy streams of α-particles from a radioactive source at a thin sheet 100 nm thickness of gold.

Photographic film is placed around the foil. Rutherford conducted a famous experiment in which alpha particles were fired at a piece of gold foil. Ernest Rutherford performed the gold foil experiment to propose the atomic model. Rutherford model of an atom. Most of the time the alpha particles would pass through the foil without any change in their trajectories which is what was expected if JJ Thomsons.

Two of his students Hans Geiger and Ernest Marsden an undergraduate set out to measure the number of alpha particles. His experiment caused him to reject Thomsons atomic model who was. The main area on the film where the alpha particles hit the film were THROUGH the foil in a straight line. A few alpha particles were reflected by a foil leading Rutherford to the conclusion that the atom contains a small very dense region in which most of the atoms _____ is concentrated. A plum pudding was a Christmas cake studded with raisins plums.

Rutherfords conducted an experiment by bombarding a thin sheet of gold with α-particles and then studied the trajectory of these particles after their interaction with the gold foil. Five years earlier Rutherford had noticed that alpha particles beamed through a hole onto a. His experiment caused him to reject Thomsons atomic model who was. This chemistry video tutorial provides a basic introduction into Rutherfords Gold Foil Experiment. Aug 8 2014.
 Source: studylib.net
Source: studylib.net
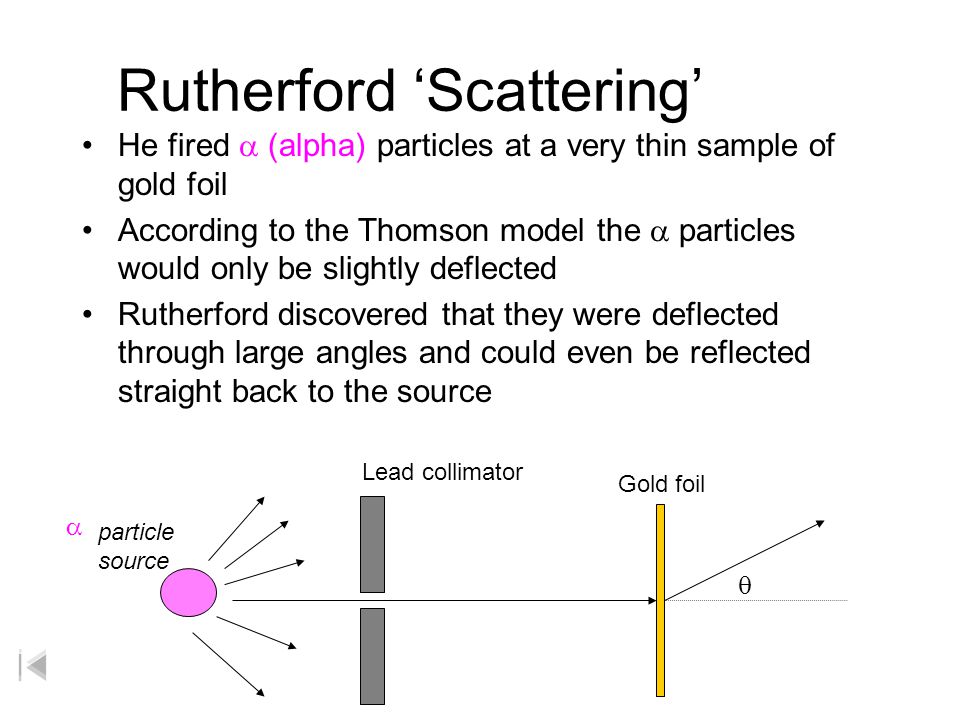
His two students Hans Geiger and Ernest Marsden directed a beam of alpha particles at a very thin gold leaf suspended. When Rutherford shot α particles through gold foil he found that most of the particles went through. His experiment caused him to reject Thomsons atomic model who was. Two of his students Hans Geiger and Ernest Marsden an undergraduate set out to measure the number of alpha particles. Rutherford overturned Thomsons model in 1911 with his famous gold-foil experiment in which he demonstrated that the atom has a tiny massive nucleus.
 Source: slideplayer.com
Source: slideplayer.com
Describe Rutherfords gold foil experiment and explain how this experiment altered the plum pudding model. Alpha particles are fired at thin gold foil. Rutherford conducted a famous experiment in which alpha particles were fired at a piece of gold foil. Rutherford model of an atom. A plum pudding was a Christmas cake studded with raisins plums.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
The existence of protons was also known as was the fact that atoms were neutral in charge. Ernest Rutherford performed the gold foil experiment to propose the atomic model. Other areas were scattered around the film and even back by the alpha particle source. Here are the steps that were completed. Aug 8 2014.
 Source: slideshare.net
Source: slideshare.net
So think of the model as a spherical Christmas cake. Use the microscope to observe different parts of the screen and look for. Describe Rutherfords gold foil experiment and explain how this experiment altered the plum pudding model. In 1905 Ernest Rutherford did an experiment to test the plum pudding model. Some scattered in various directions and a few were even deflected back towards the source.
 Source: byjus.com
Source: byjus.com
Rutherford and his co-workers made a fundamental contribution in understanding the structure of the atom and establishing the presence of a small nucleus in the atom. Use the microscope to observe different parts of the screen and look for. Select all the statements that correctly describe the observations made. Two of his students Hans Geiger and Ernest Marsden an undergraduate set out to measure the number of alpha particles. Opposite the gold foil is a zinc sulfide screen that emits a flash of light when struck by an alpha particle.
 Source: pinterest.com
Source: pinterest.com
Other areas were scattered around the film and even back by the alpha particle source. The Rutherford Scattering Experiment. The foundations of modern ideas about atomic structure are considered to have been laid by Sir Ernest Rutherford in 1911 with his postulates concerning the scattering of alpha particles by atoms. Most of the beams went through the foil but a few were deflected. He beamed a ray of alpha particles onto a gold foil and.

Describe Rutherfords gold foil experiment. Opposite the gold foil is a zinc sulfide screen that emits a flash of light when struck by an alpha particle. Most of the beams went through the foil but a few were deflected. Rutherford conducted a famous experiment in which alpha particles were fired at a piece of gold foil. Rutherford and his co-workers made a fundamental contribution in understanding the structure of the atom and establishing the presence of a small nucleus in the atom.
 Source: tec-science.com
Source: tec-science.com
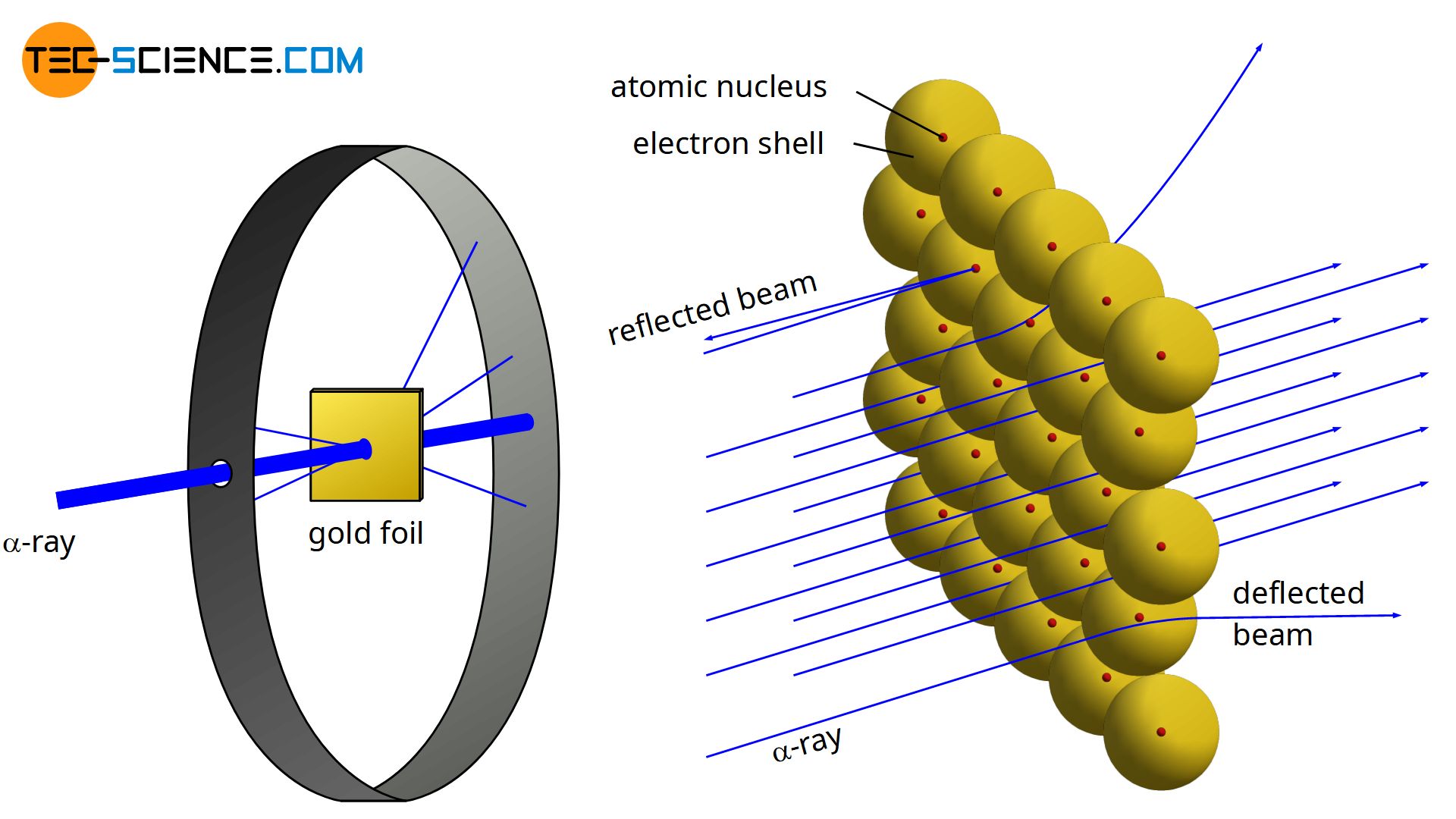
The tutorial simulates diffraction of alpha particles helium nuclei containing two positive charges by a thin foil made of gold metal. By bombarding a very thin gold foil with alpha particles Hans Geiger and Ernest Marsden both students of Rutherford observed that a small fraction 1 in 8000 of these particles were deflected at large angle as if it bounced off a heavy obstacle. To operate the tutorial use the slider to increase the slit width from. Rutherfords gold foil experiment Rutherfords alpha particle scattering experiment refers. Point the alpha particles being created by the alpha source at the piece of gold foil.
 Source: zapscience.com
Source: zapscience.com
Other areas were scattered around the film and even back by the alpha particle source. Describe Rutherfords gold foil experiment. Rutherfords gold foil experiment was very important in developing the nuclear model of the atom. Alpha particles are fired at thin gold foil. A plum pudding was a Christmas cake studded with raisins plums.
 Source: youtube.com
Source: youtube.com
Rutherfords gold foil experiment Rutherfords alpha particle scattering experiment refers. The Rutherford Scattering Experiment. In 1905 Ernest Rutherford did an experiment to test the plum pudding model. Since the intact atom had no net charge and the electron and proton had opposite charges the next step after the discovery of subatomic particles. Rutherfords gold foil experiment Rutherfords alpha particle scattering experiment refers.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title describe rutherfords gold foil experiment by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






