Your Gold lewis dot structure images are ready in this website. Gold lewis dot structure are a topic that is being searched for and liked by netizens today. You can Find and Download the Gold lewis dot structure files here. Download all free vectors.
If you’re looking for gold lewis dot structure pictures information connected with to the gold lewis dot structure interest, you have visit the ideal blog. Our website frequently gives you hints for seeing the maximum quality video and picture content, please kindly search and locate more enlightening video content and images that match your interests.
Gold Lewis Dot Structure. Many elements do not follow the octet rule. Molecular structure in which the valency electrons are shown as dots so placed between the bonded atoms that one pair of dots represents two electrons or one covalent single bond eg. Find the total valence electrons for the molecule. Lewis dot structure is the classical bonding model in which only valence electrons of the atoms are used.
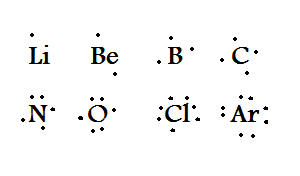 Year 11 Misadventures Electron Dot Diagrams From year11misadventures.blogspot.com
Year 11 Misadventures Electron Dot Diagrams From year11misadventures.blogspot.com
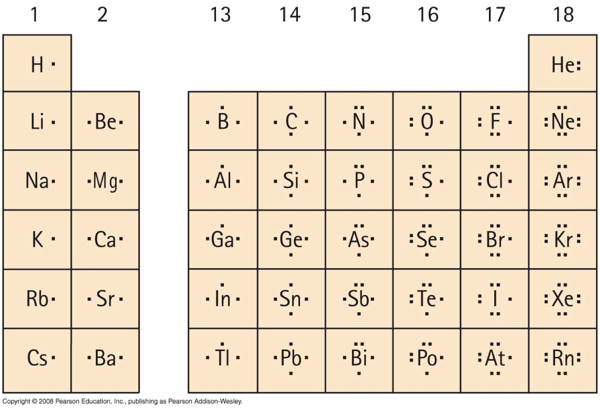
Index Chemical concepts Chemistry of the Elements Periodic Table. When this situation occurs the molecules Lewis structure is said to be a resonance structure and the molecule exists as a resonance hybrid. Predicted data is generated using the ACDLabs Percepta Platform - PhysChem Module. Lewis-Dot Diagrams Dots are placed one at a time on the four sides of the symbol then paired until all valence electrons are used Maximum of. Lewis defined a base as an electron pair donor and an acid as an electron pair acceptor. Since nickel is a transition element you have to manually write out its electron configuration and figure out how many electrons the l.
Lewis Electron Dot Structure Of Gold DIAGRAM Electron Dot Diagram Gold FULL Version HD Introduction to Lewis Structures for Covalent Molecules Exceptions to the Octet Rule Lewis Electron Dot Structures CK 12 Foundation.
Lewis-Dot Diagrams Dots are placed one at a time on the four sides of the symbol then paired until all valence electrons are used Maximum of. Many elements do not follow the octet rule. Lewis-Dot Diagrams Lewis Dot Diagrams are a way to represent the valence electrons in an atom. Elements symbol represents the nucleus and inner-level electrons Dots represent the valence electrons 53 Electron Configuration. Some of the exceptions about octet rule are given below. Complete octets on outside atoms.
 Source: researchgate.net
Source: researchgate.net
Molecular structure in which the valency electrons are shown as dots so placed between the bonded atoms that one pair of dots represents two electrons or one covalent single bond eg. Now we build Lewis structures by elaborating from neutral atoms and of course we have to account for the charge on the atom or radical ion. Steps for Writing Lewis Structures. An electron or molecule which contains unpaired electrons in its outermost shell or valence shell is considered as free radical. Lewis structures are a useful way to.
 Source: goconqr.com
Source: goconqr.com
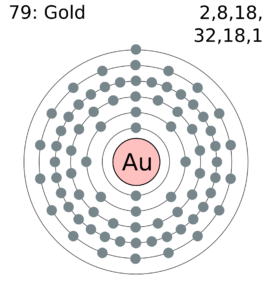
Golds Name in Other Languages. Electron Configuration into Shells. The Lewis Dot Structure is a visual which represents the outermost shell of electrons also known as valence electrons and possible covalent bonds within an atom or molecule. It is shown below with the help of Lewis dot structure. The Lewis structure would look like.
 Source: periodicnetwork2013tag.pbworks.com
Source: periodicnetwork2013tag.pbworks.com
254 C LabNetwork old LN00202325. Complete octets on outside atoms. Lewis Electron Dot Structure Of Gold DIAGRAM Electron Dot Diagram Gold FULL Version HD Introduction to Lewis Structures for Covalent Molecules Exceptions to the Octet Rule Lewis Electron Dot Structures CK 12 Foundation. You cant simply draw a Lewis dot structure for a metal you have to know the metal. A double bond is represented by two pairs of dots.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Lewis dot structures are commonly referred to as electron dot structures or Lewis structures. Since nickel is a transition element you have to manually write out its electron configuration and figure out how many electrons the l. 254 C LabNetwork old LN00202325. It is shown below with the help of Lewis dot structure. Overview of Gold.
 Source: goldelementproject1.weebly.com
Source: goldelementproject1.weebly.com
Many elements do not follow the octet rule. If the theoretical calculations are done carefully we. Lewis dot structure is the classical bonding model in which only valence electrons of the atoms are used. Now we build Lewis structures by elaborating from neutral atoms and of course we have to account for the charge on the atom or radical ion. H always goes outside.
 Source: periodictable.me
Source: periodictable.me
These valence electrons are negatively charged and are attracted to the positively charged nucleus made up of neutrons and protons. Take for example nitrate ion N O 3. Nitrogen Group V has 5 valence electrons. Lewis structures are a useful way to. Lewis-Dot Diagrams Lewis Dot Diagrams are a way to represent the valence electrons in an atom.
 Source: chemistryofmaterials2013.wikidot.com
Source: chemistryofmaterials2013.wikidot.com
And we throw in another electron so that we have 5 3 6 1 24 valence. Answer 1 of 2. Predicted data is generated using the ACDLabs Percepta Platform - PhysChem Module. Exceptions to the Octet Rule. Since nickel is a transition element you have to manually write out its electron configuration and figure out how many electrons the l.
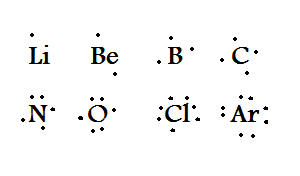 Source: year11misadventures.blogspot.com
Source: year11misadventures.blogspot.com
Instead of acting like an entitled egomaniac who thinks hes the smartest guy in the room like the other guy did Ill provide an actual answer. Cu If you had a pair of coppers you would write CuCu Gold is essentially the same. Predicted data is generated using the ACDLabs Percepta Platform - PhysChem Module. There are 2 lone pairs of electrons on each O atom and As has 1 lone pair of electrons on each atom. We should verify the usefulness of our simple predictions with molecular orbital theory.
 Source: youtube.com
Source: youtube.com
Lewis dot structures reflect the electronic structures of the elements including how the electrons are paired. Lewis Dot Diagrams of Selected Elements. Put two electrons between atoms to form a chemical bond. Lewis dot structures reflect the electronic structures of the elements including how the electrons are paired. Many elements do not follow the octet rule.
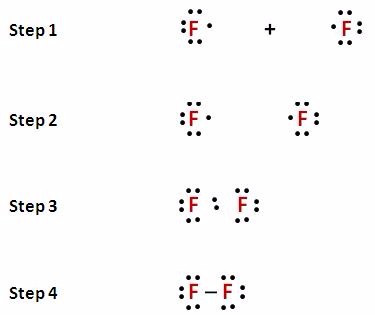
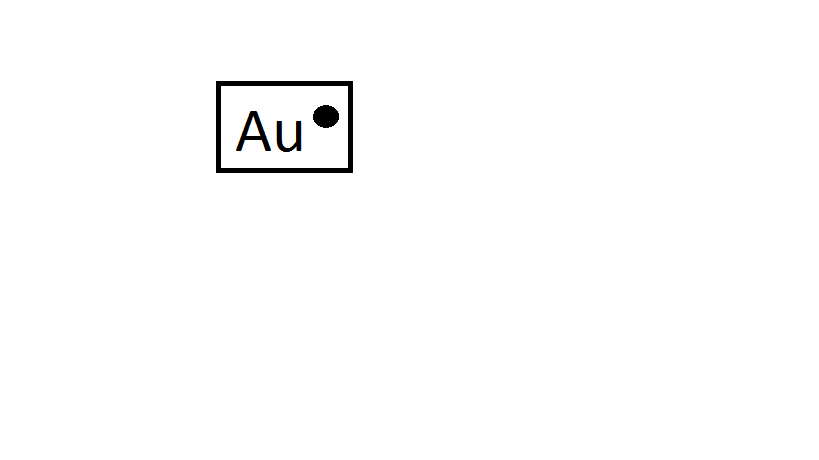
Cu If you had a pair of coppers you would write CuCu Gold is essentially the same. Lewis dot structures are commonly referred to as electron dot structures or Lewis structures. And we throw in another electron so that we have 5 3 6 1 24 valence. These electrons are less stable and do not obey the. You cant simply draw a Lewis dot structure for a metal you have to know the metal.
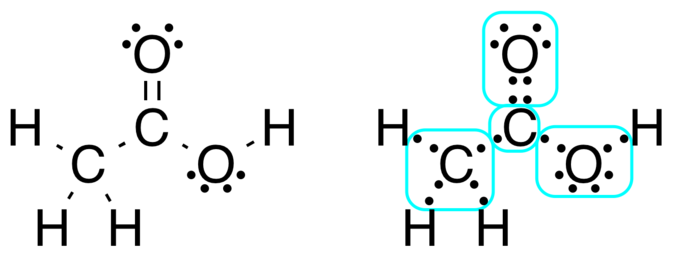 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Lewis Dot Diagrams of Selected Elements. There are 2 lone pairs of electrons on each O atom and As has 1 lone pair of electrons on each atom. Index Chemical concepts Chemistry of the Elements Periodic Table. Lewis structures are a useful way to. The Lewis Dot Structure is a visual which represents the outermost shell of electrons also known as valence electrons and possible covalent bonds within an atom or molecule.

In the case of copper the electron from the s shell is moved to fill the d shell so there is only one electron in the s shell. Many elements do not follow the octet rule. What is the electron dot diagram for carbon. You cant simply draw a Lewis dot structure for a metal you have to know the metal. These theories which include Lewis structures VSEPR and hybridization are simple models that help predict chemical properties.
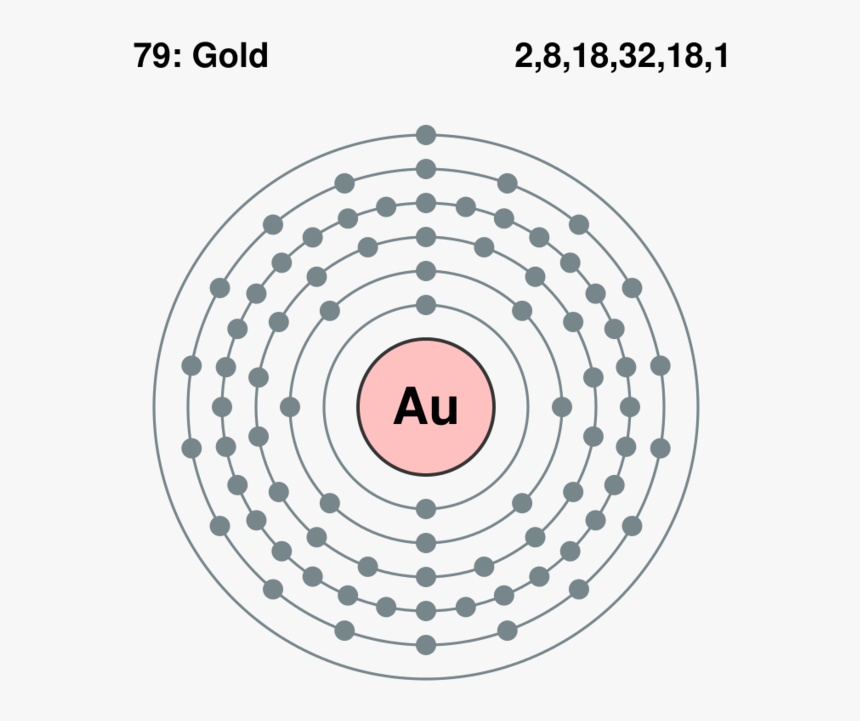 Source: kindpng.com
Source: kindpng.com
H always goes outside. Atomic Structure of Gold. Lewis dot structures reflect the electronic structures of the elements including how the electrons are paired. These valence electrons are negatively charged and are attracted to the positively charged nucleus made up of neutrons and protons. You cant simply draw a Lewis dot structure for a metal you have to know the metal.

Steps for Writing Lewis Structures. Index Chemical concepts Chemistry of the Elements Periodic Table. However Lewis dot structures and hybridization are approximations that may or may not match reality. Lewis structure is very important in chemistry because they are used in many important concepts of general chemistry such as chemical bonding resonance valence shell electron pair repulsion theory prediction of. There are 2 lone pairs of electrons on each O atom and As has 1 lone pair of electrons on each atom.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Oxygen Group VI has 6 valence electrons. Molecular structure in which the valency electrons are shown as dots so placed between the bonded atoms that one pair of dots represents two electrons or one covalent single bond eg. Lewis dot structure is the classical bonding model in which only valence electrons of the atoms are used. Since nickel is a transition element you have to manually write out its electron configuration and figure out how many electrons the l. There are 2 lone pairs of electrons on each O atom and As has 1 lone pair of electrons on each atom.
 Source: youtube.com
Source: youtube.com
There are 2 lone pairs of electrons on each O atom and As has 1 lone pair of electrons on each atom. Electron Distributions Into Shells for the First Three Periods. Lewis dot structures reflect the electronic structures of the elements including how the electrons are paired. CH 4 NH 3 I 2. Lewis dot structure is the classical bonding model in which only valence electrons of the atoms are used.
 Source: byjus.com
Source: byjus.com
Answer 1 of 2. H 2 S NCl 3 OH-Put the least electronegative atom in the center. Atomic Structure of Gold. Molecular structure in which the valency electrons are shown as dots so placed between the bonded atoms that one pair of dots represents two electrons or one covalent single bond eg. Put two electrons between atoms to form a chemical bond.
 Source: hms2014gold.weebly.com
Source: hms2014gold.weebly.com
Some of the exceptions about octet rule are given below. In the case of copper the electron from the s shell is moved to fill the d shell so there is only one electron in the s shell. Index Chemical concepts Chemistry of the Elements Periodic Table. You cant simply draw a Lewis dot structure for a metal you have to know the metal. Lewis dot structure is the classical bonding model in which only valence electrons of the atoms are used.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title gold lewis dot structure by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






