Your Specific heat capacity of gold images are ready. Specific heat capacity of gold are a topic that is being searched for and liked by netizens now. You can Download the Specific heat capacity of gold files here. Download all royalty-free photos.
If you’re searching for specific heat capacity of gold images information related to the specific heat capacity of gold keyword, you have come to the right blog. Our site frequently provides you with hints for seeing the highest quality video and image content, please kindly search and find more enlightening video content and images that match your interests.
Specific Heat Capacity Of Gold. Specific heat or specific heat capacity is a. Mass times the change in temperature divided by the heat added. The specific heat of gold is given at a temperature of 0 C. Gold has a specific heat of 0129 JgC.
 What Is Specific Heat Capacity Definition Formula Equation From guyhowto.com
What Is Specific Heat Capacity Definition Formula Equation From guyhowto.com
Notes on the Specific Heat of particular elements. 890 J180 g x 250C specific heat 0199 JgC. Specific heat is the amount of heat energy needed to raise the temperature of one unit of mass by 1 degree Celsius. Specific heat of Gold is 0128 Jg K. Gold has a specific heat capacity of 013 JgºC. The specific heat of gold is given at a temperature of 0 C.
It takes 4172 J to heat a piece of gold weighing 1869 g from 100 C to 270 C.
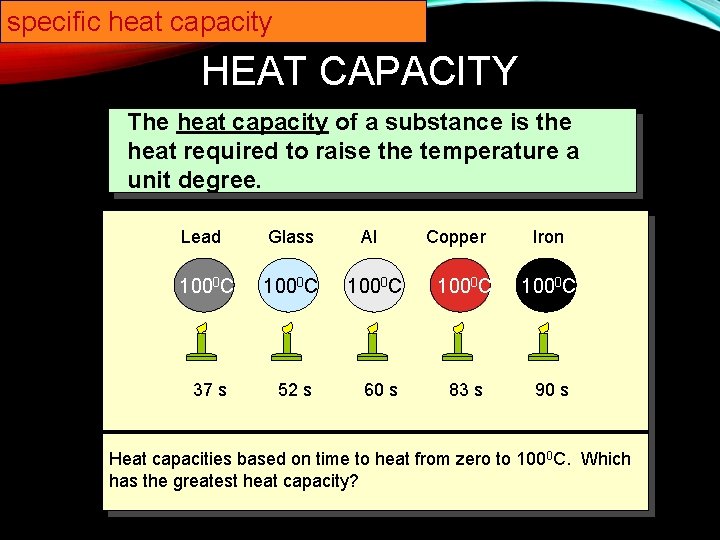
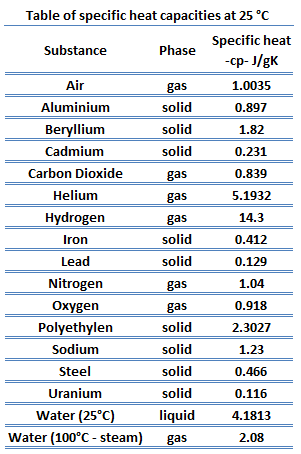
51 rows The specific heat capacity of materials ranging from Water to Uranium has. Value given for solid graphite form. Now look at your periodic table and choose a metal that is most likely the identity of the sample. Al 0903 JgC Pb 0160 JgC The metals are added to two insulated cups or calorimeters each containing the same amount of water initially at room temperature. Specific heat of Gold is 0128 Jg K. What does it mean that gold has a specific heat capacity of 0135 jgc.
 Source: slidetodoc.com
Source: slidetodoc.com
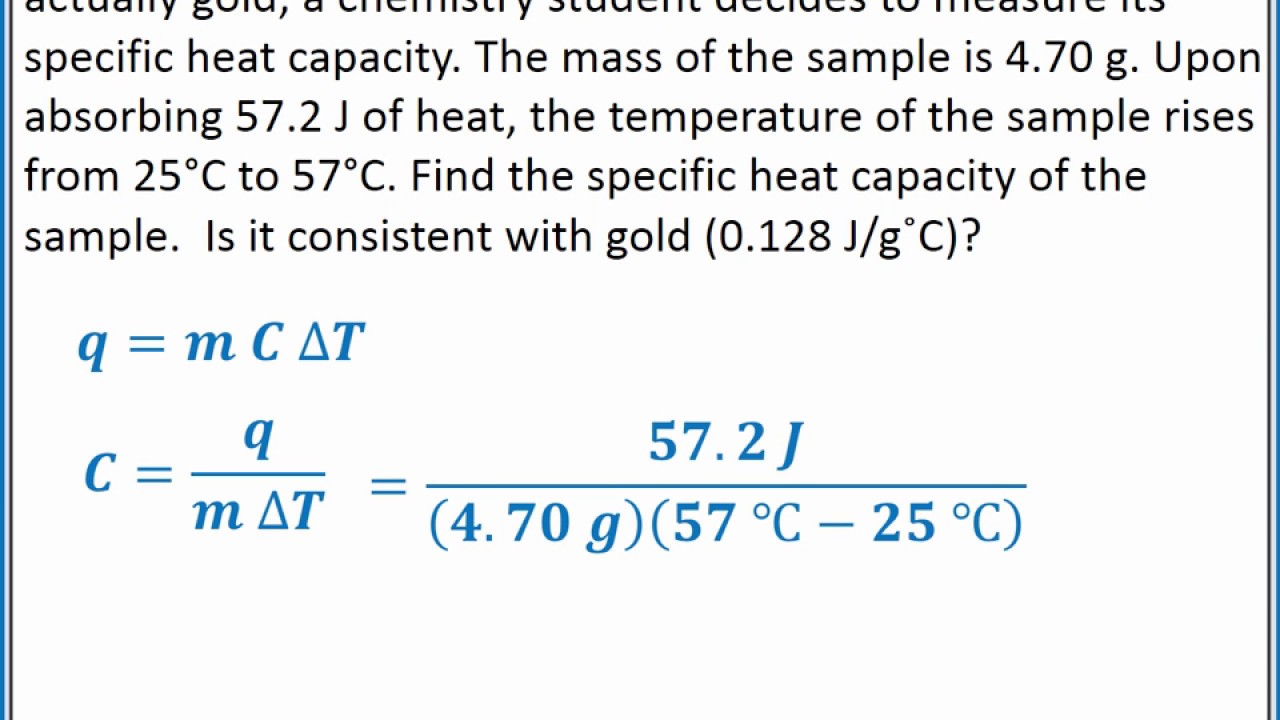
Calculate the specific heat capacity of gold in calg C. Given that the specific heat of gold is 0129 JgºC calculate the final system temperature if a 2000 g block of gold at 1000 ºC is placed in a coffee-cup calorimeter containing 500 g of water at an initial temperature of 250 ºC. Hafnium Specific heat capacity of Hafnium. ΔT difference between the final and initial temperatures of the substance. Q m c Δ T b.
 Source: youtube.com
Source: youtube.com
Value given for solid phase. Value given for solid phase. Value given for gas phase of H 2 Helium. In this problem since the initial temperature of water lower than that of the initial temperature of gold thus when they came in contact with each other the heat from the gold would transfer into the water. Hafnium Specific heat capacity of Hafnium.
 Source: cpanhd.sitehost.iu.edu
Source: cpanhd.sitehost.iu.edu
What is the final temperature of the water and pieces of metal. Recall that the heat capacity of water is 4184 Jg C. Notes on the Specific Heat of particular elements. Q m c Δ T b. Value given for solid graphite form.
 Source: britannica.com
Source: britannica.com
Specific heat capacity of gold C is 0129 kJ kgK. The specific heat capacity is the amount of heat it takes to change. Now look at your periodic table and choose a metal that is most likely the identity of the sample. Determine if its endothermic or exothermic 1. A 315 g wafer of pure gold initially at 697 oC is submerged into 636 g.
 Source: slidetodoc.com
Source: slidetodoc.com
100 gram blocks of both aluminum and gold are left outside in the sun for 15 minutes. Specific heat capacity of gold C is 0129 kJ kgK. The specific heat capacity of gold is 0128 JgC. The value of the specific heat of a material is given by the following formula. How many joules of heat energy are required to raise the temperature of 15 grams of gold from 22 C to 85 C.
 Source: pinterest.com
Source: pinterest.com
A table of some. Al 0903 JgC Pb 0160 JgC The metals are added to two insulated cups or calorimeters each containing the same amount of water initially at room temperature. The specific heat of gold is given at a temperature of 0 C. An unknown metal has a mass of 180 g. Latent Heat of Fusion of Gold is 1255 kJmol.
 Source: material-properties.org
Source: material-properties.org
An unknown metal has a mass of 180 g. Value given for gas phase of O. 71 rows The relationship between heat and temperature change is usually expressed in the form. What does it mean that gold has a specific heat capacity of 0135 jgc. Specific heat capacity of gold C is 0129 kJ kgK.
 Source: pinterest.com
Source: pinterest.com
The specific heat of gold is given at a temperature of 0 C. Determine if its endothermic or exothermic 1. Specific heat capacity of Incoloy 600. Specific heat or specific heat capacity is a. Specific heat capacity of Gold.
 Source: researchgate.net
Source: researchgate.net
Value given for gas phase. An unknown metal has a mass of 180 g. The specific heat capacity of gold is 0128 JgC. 71 rows The relationship between heat and temperature change is usually expressed in the form. The relation between S and C is C mass of obect x specific heat of object.
 Source: slidetodoc.com
Source: slidetodoc.com
The relation between S and C is C mass of obect x specific heat of object. The value of the specific heat of a material is given by the following formula. Specific heat capacity of Incoloy 600. Value given for solid graphite form. The specific heat capacity of gold is 013 Jg C.
 Source: pinterest.com
Source: pinterest.com
Fusion and Evaporation Heat of common Materials - Melting points heat of fusions boiling points and heat to evaporate. What is the specific heat capacity of the gold. If the temperature of the metal sample rises from 150C to 400C as the sample absorbs 890 J of heat what is the specific heat of the sample. Q m c Δ T b. A 315 g wafer of pure gold initially at 697 oC is submerged into 636 g.
 Source: aplustopper.com
Source: aplustopper.com
Copper Binary Eutectic Alloys - Melting Points - Cu - Copper - binary eutectic alloys and their melting points. 890 J180 g x 250C specific heat 0199 JgC. What is the specific heat capacity of gold. Previous In an experiment the hypothesis is if the wavelength of the light shining on a plant is shortened the rate. How many joules of heat energy are required to raise the temperature of 15 grams of gold from 22 C to 85 C.
 Source: guyhowto.com
Source: guyhowto.com
Value given for solid graphite form. If the temperature of the metal sample rises from 150C to 400C as the sample absorbs 890 J of heat what is the specific heat of the sample. The molar heat capacity of iron and gold are 2519 J mol 1 C 1 and 2541 J mol 1 C 1 respectively. What does it mean that gold has a specific heat capacity of 0135 jgc. Calculate the specific heat capacity of gold in calg C.
 Source: pinterest.com
Source: pinterest.com
Specific heat capacity of Incoloy 600. Value given for gas phase. The relation between S and C is C mass of obect x specific heat of object. If the final temperature of the water was 462 C what is the specific heat capacity of the rock. It takes 4172 J to heat a piece of gold weighing 1869 g from 100 C to 270 C.
 Source: youtube.com
Source: youtube.com
71 rows The relationship between heat and temperature change is usually expressed in the form. The specific heat capacity of gold is 0128 JgC. Value given for gas phase. Specific heat capacity of gold C is 0129 kJ kgK. A table of some.
 Source: pinterest.com
Source: pinterest.com
Value given for gas phase of N 2 Oxygen. Specific heat capacity of Incoloy 600. Gold at 25 degrees Celsius has a specific heat of 0129 joules per gram per degree Celsius. Value given for solid graphite form. Heat is a combination of kinetic energy measured by temperature and potential energy.
 Source: slidetodoc.com
Source: slidetodoc.com
Calculate the specific heat capacity of gold in calg C. Gold has a specific heat capacity of 013 JgºC. 100 gram blocks of both aluminum and gold are left outside in the sun for 15 minutes. Copper Binary Eutectic Alloys - Melting Points - Cu - Copper - binary eutectic alloys and their melting points. Latent Heat of Fusion of Gold is 1255 kJmol.
 Source: theengineeringmindset.com
Source: theengineeringmindset.com
A 315 g wafer of pure gold initially at 697 oC is submerged into 636 g. What is the final temperature of the water and pieces of metal. Next added to sizable financial capacity we also infer managements increased confidence in underlying. How many joules of heat energy are required to raise the temperature of 15 grams of gold from 22 C to 85 C. 51 rows The specific heat capacity of materials ranging from Water to Uranium has.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title specific heat capacity of gold by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






